Messenger RNA vaccines proved effective during the COVID-19 pandemic. Ways to further optimize the encoded proteins have remained an ongoing pursuit. Protein nanoparticle immunogens, which can display multiple antigen copies in precise arrays, amplify antibody responses by clustering B cell receptors. Integrating these two approaches could yield vaccines that trigger both strong antibody and T cell immunity while retaining the speed and scalability of mRNA production.
In the study, “Computationally designed mRNA-launched protein nanoparticle immunogens elicit protective antibody and T cell responses in mice,” published in Science Translational Medicine, researchers genetically fused a stabilized SARS-CoV-2 receptor binding domain variant, Rpk9, to a computationally optimized 60-subunit scaffold nanoparticle, I3-01NS.
mRNA-launched RBD nanoparticles outperformed the comparator mRNA vaccines across experiments. In the single-dose Wuhan-Hu-1 arm, Rpk9–I3-01NS mRNA elicited ~28× higher titers than membrane-anchored S-2P mRNA and ~11× higher than secreted RBD-trimer mRNA.
Even the lowest mRNA dose produced responses comparable to or greater than those achieved with substantially higher doses of standard spike-encoding formulations. Serum analyses after boosting showed persistent neutralization against Wuhan-Hu-1, while additional assays demonstrated cross-reactivity to omicron BA.5.


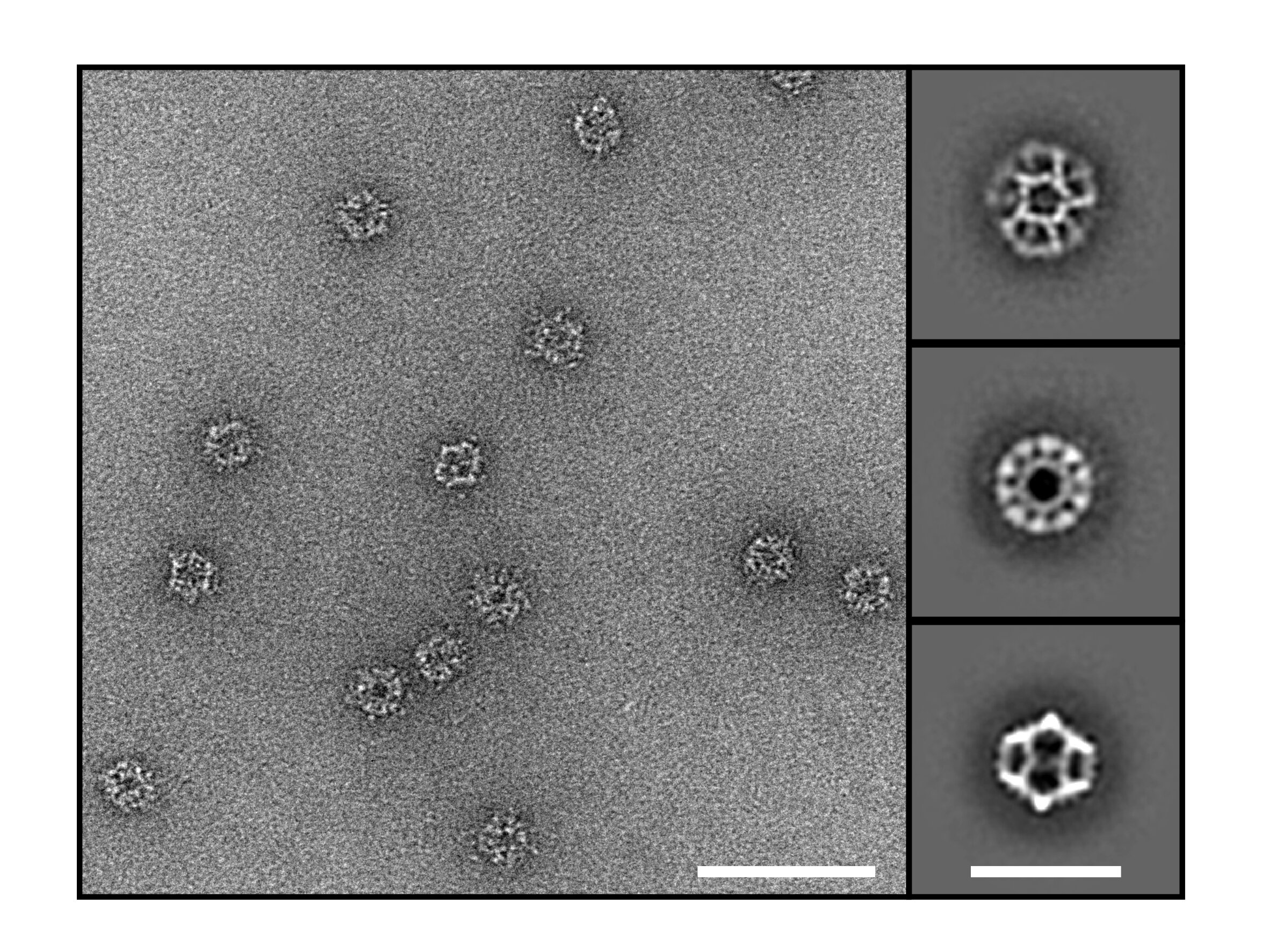
I think the key thing here is that in addition to mRNA encoding the antigen, they are also delivering mRNA for spike protein receptor in a scaffold that helps assemble the antigen into protein nanoparticles. So ultimately, the antigen will be presented in a more virus-like fashion.