Alright science nerds how cold does the freezer actually have to be to get a jagerpop that is consistent?
tl;dr Around -21°C (-9°F). Realistically probably a tad lower than that.
Formula for freezing point depression is:
ΔTf = i * Kf * m
ΔTf = freezing point depression in absolute degrees (C or K)
i = van 't Hoff factor, the number of particles the solutes splits into, ethanol does not split, so 1
Kf = freezing point suppression constant, for water 1.86°C * kg / mol
m = molality of the solution (aka how much you add), looked it up and it’s 11.42 mol for 40% ethanol/water mixture
1 * 1.86 * 11.42 = 21.2412°CIn reality, Jägermeister is not a pure water/ethanol mixture and all that other stuff in there drops the freezing point by a bit as well. Ethanol is the biggest contributor tho. So maybe add 1° or 2° to this.
Is Jägermeister really that green in the US?
The bottle is green, the liquid is brown
Yeah, that’s how it is here as well, so these Jägersicles look fake.
Food coloring
No, at -15 it turns green /s
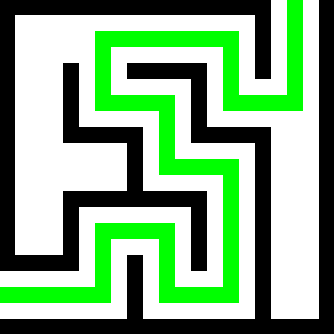
I did not know about that formula, very cool. It seems linear (unless the molality is a non-linear term), whereas the empirical data gets pretty whacky at higher concentrations. Maybe its validity is for low concentrations? I’m getting closer to -27C from this plot

Either way that’s getting close to “don’t lick it” temperatures lol!
The figure you’ve linked plots concentration by weight (wt.%), while the alcohol content of drinks is usually given in volume percent (v/v). Ethanol is less dense than water, so a 30% concentration by weight is a higher concentration by volume.
Imagine a 100g solution of 30wt.% alcohol. That means that 30g are ethanol and 70g are water. the 70g of water translate to 70ml volume (density 1g/ml) and the 30g of ethanol translate to 30/0.789 = 38.02ml. So in total, you would have 108.02ml of liquid and the concentration of ethanol by volume would be 38.02/108.02 = 35.2%.
Why it gets wacky at the end: Ethanol freezes at -114°C, water freezes at 0°C, but at specific concentrations, the eutectic composition, the solution freezes at a lower point than either of its constituents. The eutectic point is the lowest possible freezing point of a solution. The formula I gave is not applicable to eutectic solutions and is an approximation based on perfect solutions (which in reality don’t really exist).
There is only one error in your equations (and that doesn’t change much about your point) is that the volume of that mixture is slightly lower than 108mL and should sit at about 107.1mL.
Amazing, thanks for the clarification!
All the other stuff in the drink might lower it a bit though, especially the 14% (!!!) sugar. There’s a sugar cube in every shotglass.
You’re right, I edited it.
“it has an alcohol by volume of 35% (61 degrees proof, or US 70 proof)”
Whoops. Well, I forgot to account for sugar and other stuff which decrease freezing temperature as well, so the result might still be around right. It’s an ok ballpark at least. Precisely calculating a solutions freezing temperature when it has that many different solubles is pretty hard.
Is Jägermeister really that green in the US?
It’s reddish brown everywhere else.
Wouldn’t surprise me, they do love their food dyes over there
At least this 🤏 cold. I’m pretty sure it’s mixed since pure Jägermeister isn’t green.
All those who have tried it this morning are currently busy trying to take the pop out.
How much/what’s best to mix with to get a better freezing point? Always looking for new recipes.
there’s a bar in Edinburgh Scotland called Panda and Sons, they bring the spirits to that spot where they can remove the water ice and then add other stuff or mix it up. They have their menu online that gives some details.
My wife and I sampled most of the drinks. Interesting approach but you need some skill and the right equipment to make more than a mess
Thnx, very interesting.
But… Isn’t the actual Jagermeister liquid inside the bottle actually red…?
It’s brown. Similar colour to cola.
I actually like Jaeger but can’t find what this recipe would be. I saw someone made Jaeger pops out of cranberry juice and Jaeger which sounds OK but not this color. I’m guessing they used white grape juice and a shitload of food coloring for this, assuming it’s not just a stunt for the gram
Green Booze … just like nature intended … bit late for St. Patrick’s Day … there’s always next year.
bit late for St. Patrick’s Day
german schnapps on a mostly american ‘irish’ holiday? I mean it’ll get you fucked up sure… but this example is probably food coloring because Jager is brown colored.

This… is the story… of Jägermeister